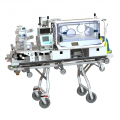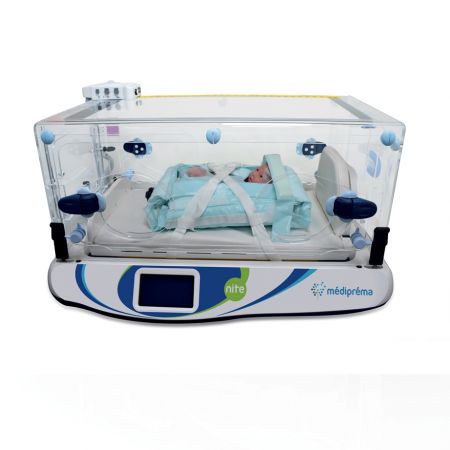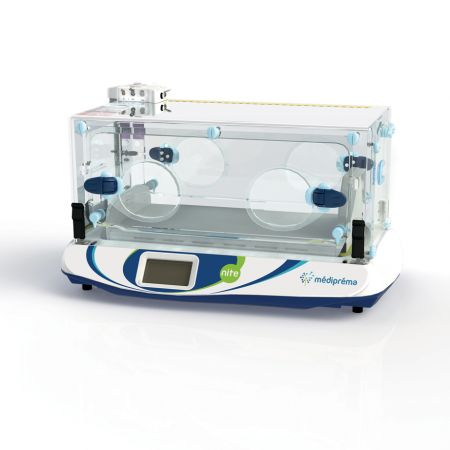
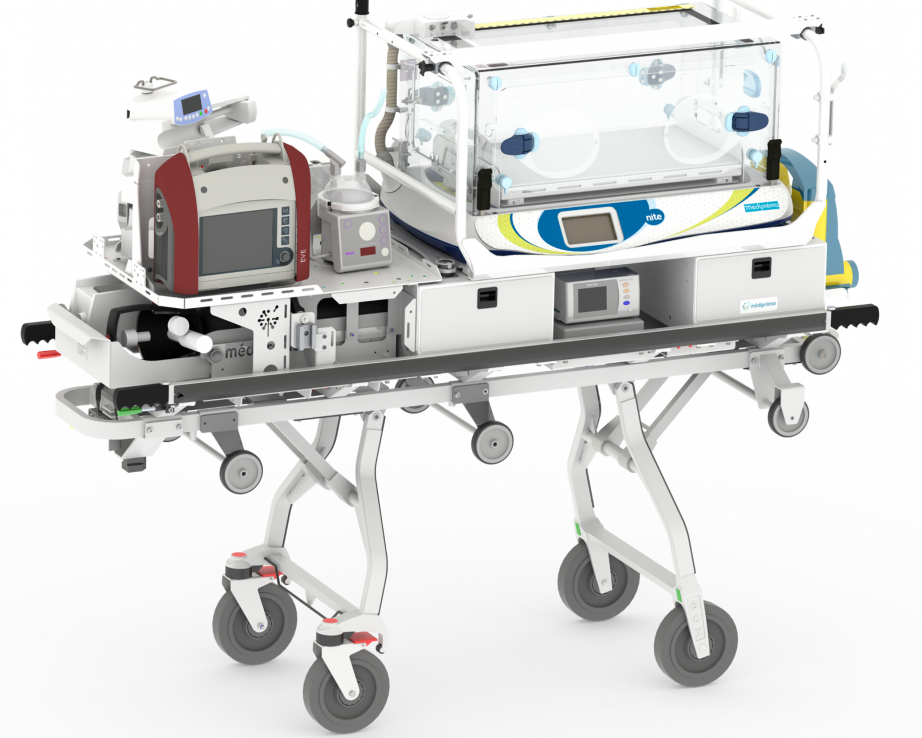
Made-to-measure transport structure Médicruiser
Flexibility
Made-to-measure solution to meet your standards, needs and budget
Reliability and safety
Each medical device is securely fixed to the assembly but remains easy to dismantle in accordance with emergency transport requirements. The structures designed by médipréma comply with European regulatory requirements related to the marketing of medical devices.
Compact, light and robust design
After sometimes more than 15 years of intensive use in difficult emergency transport conditions, médipréma structures are still in use.
All information on this product
Médipréma can produce a made-to-measure structure, based on the customer’s requirements :
- Transport types : internal transfer, road transport, helicopter or plane transport, mixed transport (interoperability between several modes of transport), etc.
- Constraints depending on the type of transport : weight, volume, onboard peripheral equipment, electricity supply and gas autonomy, etc.
- Working habits : organisation around the baby, onboard equipment, backpack, weight, working ergonomics, etc.
- Budget available for the project
An approved methodology
The design of a neonatal transport structure follows a precise process. All the solutions undergo 3D modelling to create a manufacturing plan based on the specifications requested.
Tested solutions
Médipréma has several testing rooms for the validation of its solutions. The company also works with a network of external laboratories. The Nite incubator underwent crash testing in a Cofrac laboratory to obtain EN1789 10G certification.
Certified solutions
Médipréma meets the requirements of the ISO 13485 quality system. This system is regularly audited. The company also has ISO 14001 certification, and is actively committed to a continuous improvement process for its environmental performance. All our structures are validated by CE marking. We offer our consumers complete structures validated by EN1789 10G and DO160 20G resistance calculations on transport structures.
French design and manufacture
The Nite transport incubator and all the médipréma structures are designed and manufactured at the médipréma plant, located in the Loire valley. This allows médipréma to guarantee the quality and reliability of its devices, with control of the entire production chain.
On-board systems depend on your requirements. Here are some exemples :
1. Holder for secretion aspiration system
2. Location for 2 bottles with straps
3. Shelf for accessories
4. Holder for ventilator
5. Holder for patient monitor
6. Location for syringe pumps
7. Holder for NiMH battery (not pictured)
8. Location for electric sockets
9. Air or 02 bloc
10. Holder for TPS
11. Storage box
CE marking
EN1789 10G and DO160 20G Certifications